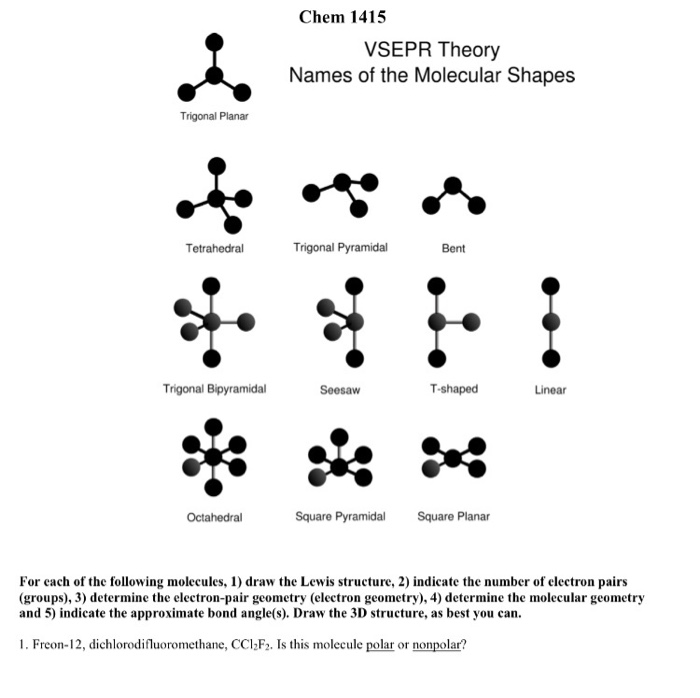
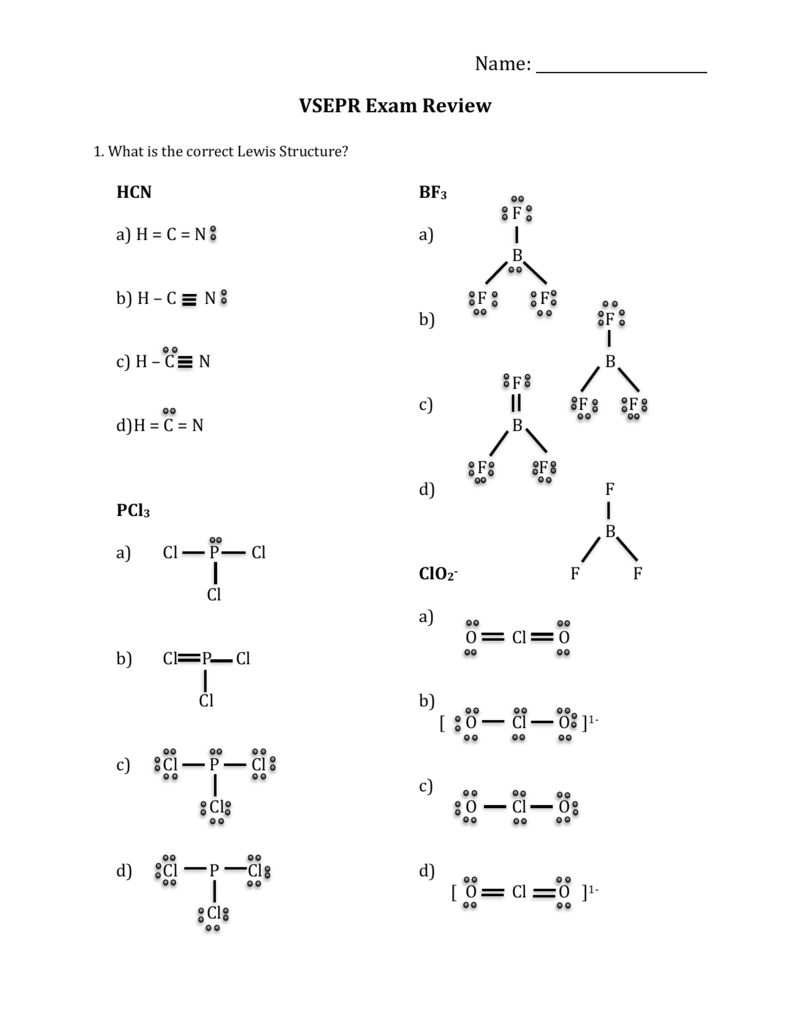
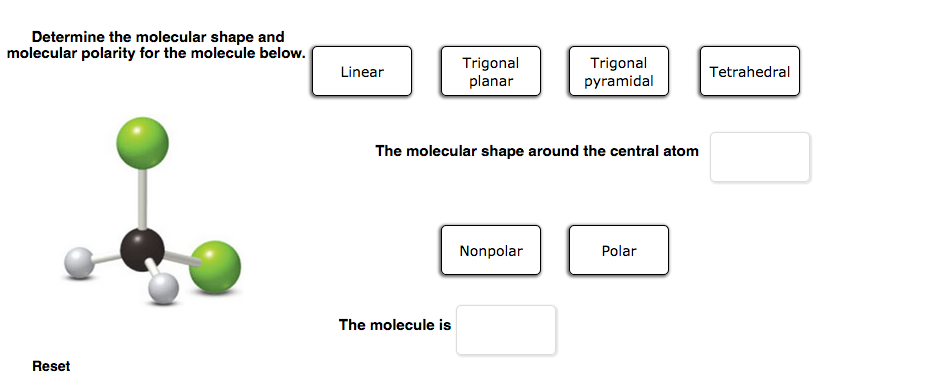
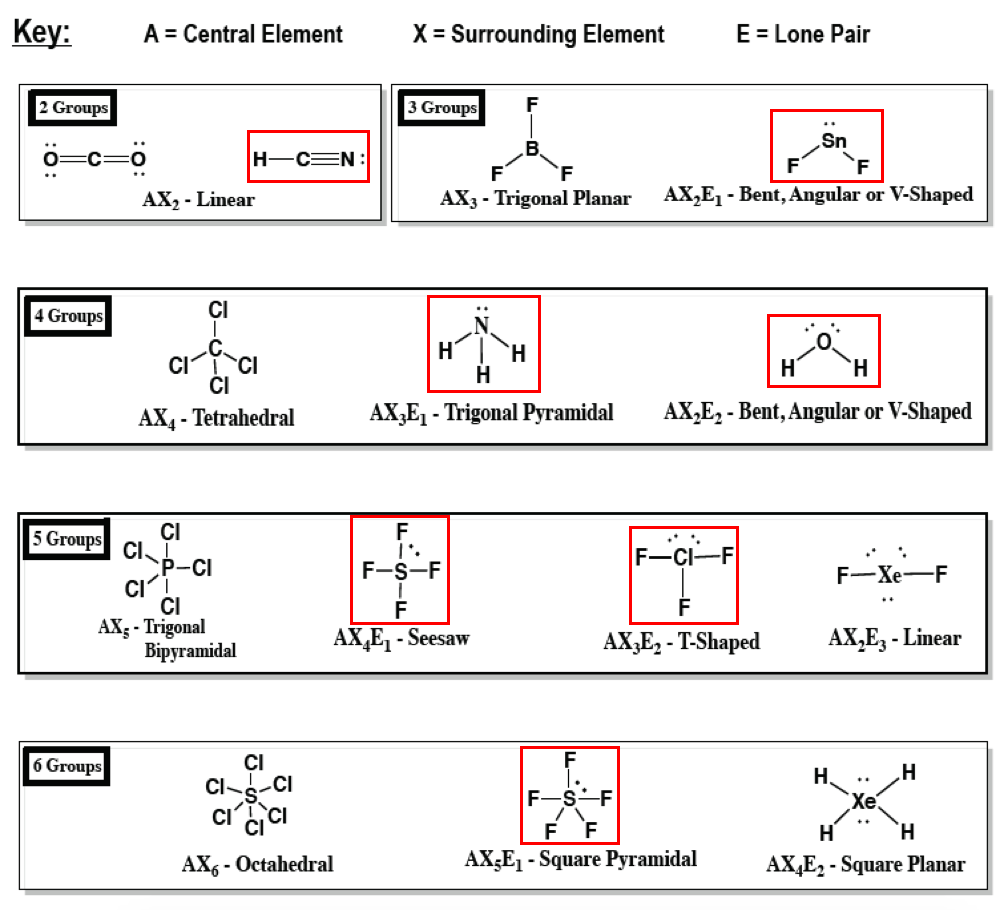
Trigonal Pyramidal Polar Or Nonpolar
Pf3 is a polar molecule.
Trigonal pyramidal polar or nonpolar. Measure of the degree of inequality in attraction of the bonding electrons to the various locations present within a molecule is known as molecular polarity. Fri sep 25 2015 10 00 am. When we talk about h3o then we concluded that the overall molecule is polar because the shape of the molecule is trigonal pyramidal which means it has the lone pair electrons due to the lone pair the force of attraction is unequal.
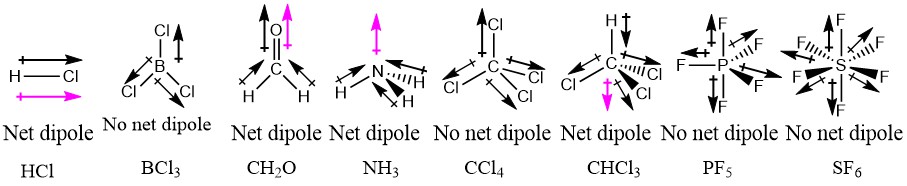
Phosphorus and fluorine have different electronegativity and the pf3 molecule also contains a lone pair. In trigonal planar the resultant of the dipole moment of two bonds can become equal. It all depends on what is attached to the central atom.
So is pf3 polar or nonpolar. Linear can be non polar as one dipole moment can cancel another when they are equal. But if they are different as in ch2o then the molecule will be quite polar.
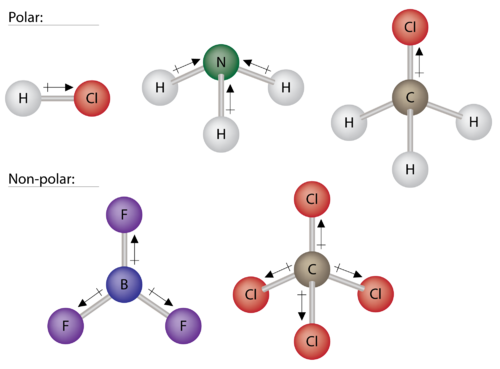
If all three atoms attached the central atom are the same then the molecule is nonpolar. A molecule with trigonal planar molecular geometry might be polar or it might not. Pcl3 polar or non polar.
It is non polar because the trigonal planar is symmetrical. Nf 2 cl molecule which has trigonal pyramidal geometry will be polar or nonpolar has to be indicated. Followings are some basic points which prove that h3o is polar.
I don t understand how they can actually cancel each other out and become non polar. As a result the shape of the molecule is trigonal pyramidal and it ensures a non zero dipole moment making the pf3 a polar molecule. So with 1 loan pair it bascially pushes the 3 bond pairs downwards.
Post by studdmuffin tue oct 20 2015 8 36 pm. Trigonal pyramidal is formed when a tetrahedral has 1 electron lone pair right. Pcl3 has a pyramidal geometry with three polar p cl bonds.
Clo3 is trigonal pyramidal and polar. The way i think of determining polarity is a continuation of geometrical theory. At this point there will be 3 dipoles pointing at different directions.
Pcl3 has a trigonal pyramidal molecular geometry and is therefore polar because the dipoles do not cancel.