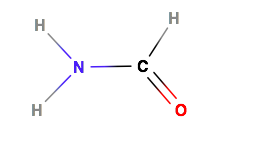
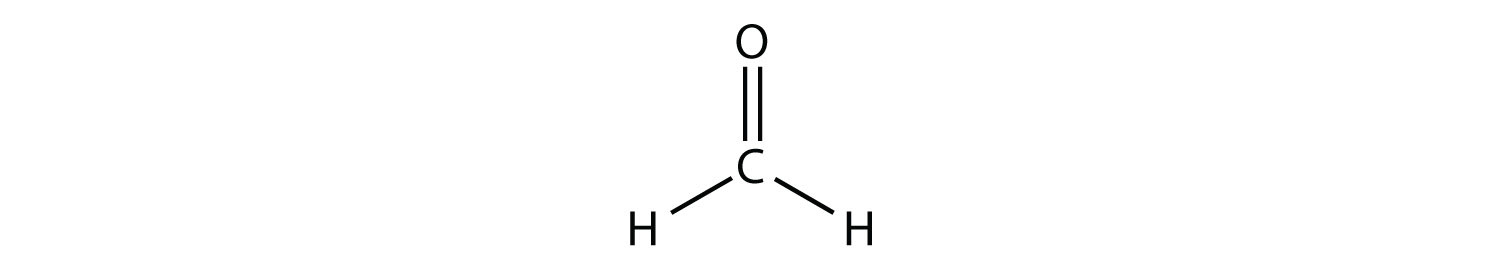
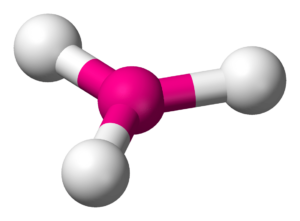
Trigonal Planar Structure
This molecule is made up of 3 equally spaced sp 2 hybrid orbitals arranged at 120 o angles.
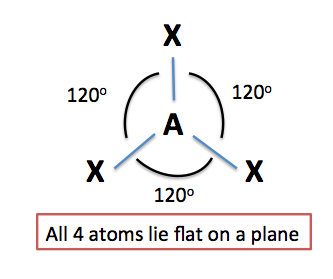
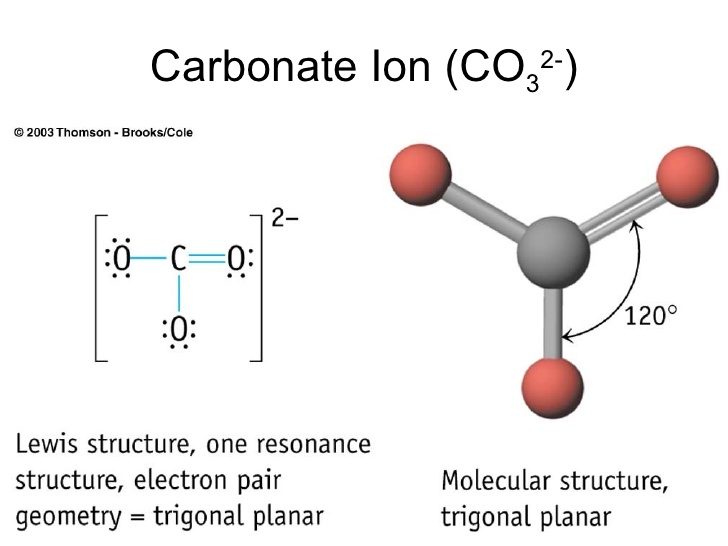
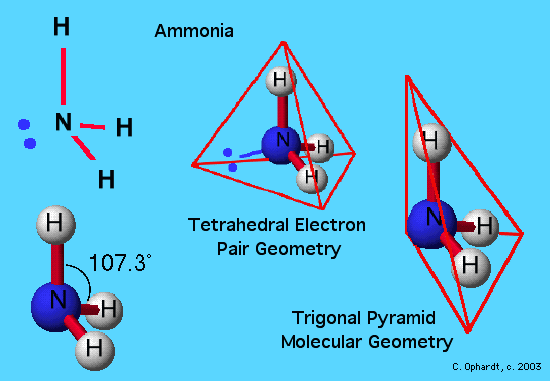
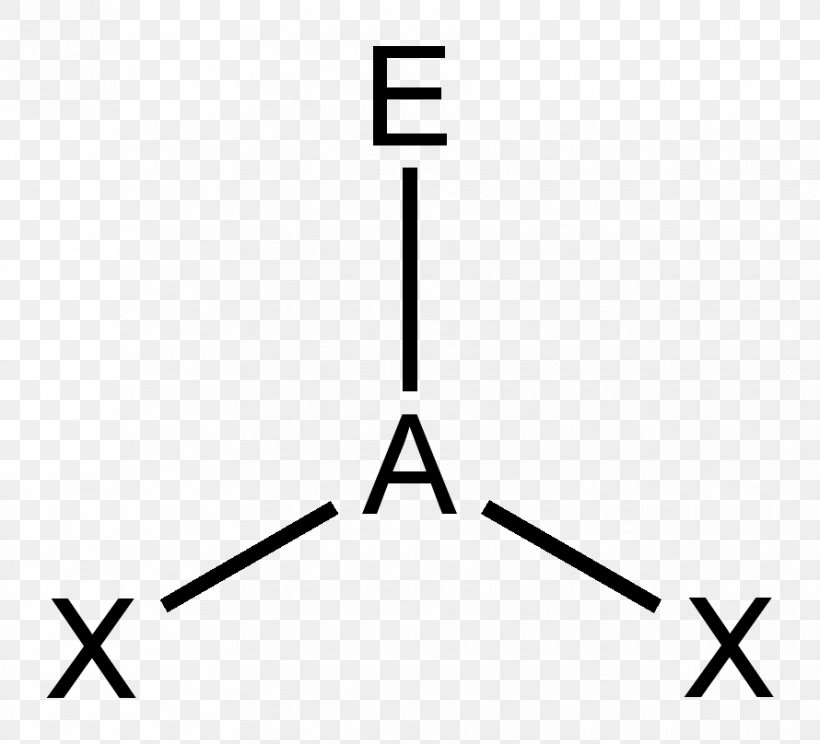
Trigonal planar structure. One is a central atom and the other three are present in the corner of the triangle as peripheral ligands. The central atom in a trigonal planar does not have a lone pair of electrons whereas the central atom in a trigonal pyramidal has one lone pair or un bonded pair of electrons. Even if the atom has a double bond it still counts as one group.
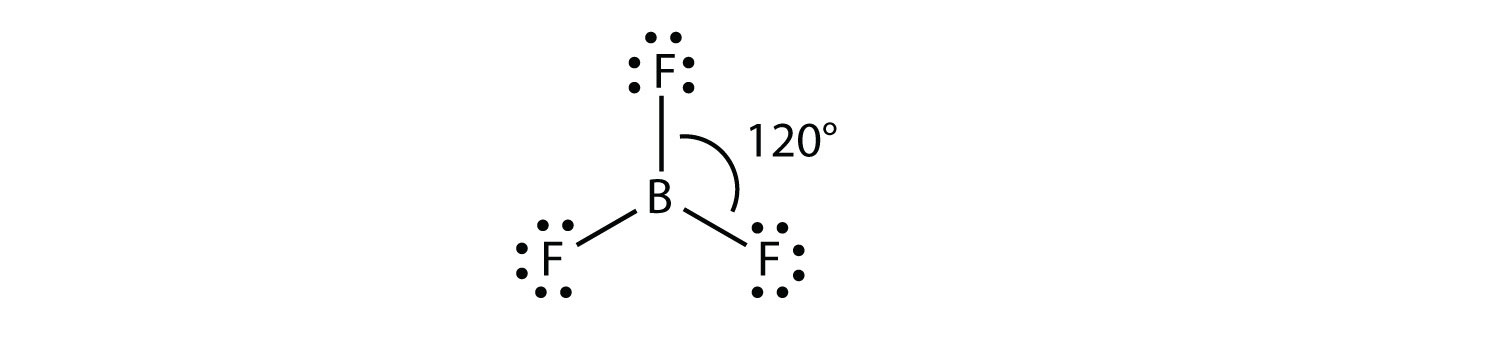
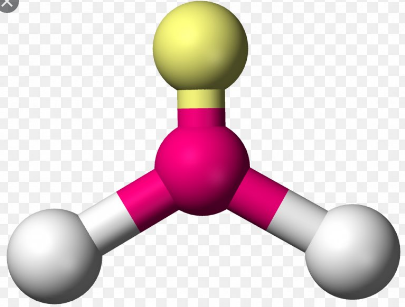
Structure of boron trifluoride an example of a molecule with trigonal planar geometry. Trigonal planar molecules have three atoms bonded to the central atom and no lone electron pairs making the steric. Trigonal planar and trigonal pyramidal are two of the several geometries that are used to describe the arrangement of atoms in a three dimensional plane.
Electrons molecular shape bf3 24 f fb f trigonal planar trigonal planar боя 18 o s 0 trigonal planar bent fgf 48 octahedral loetahedral f sf ff sta sty 34 teds 46 f s一 fs f сі el p ct seesaw trignomal bipyramidal trigonal trigonal bipyramidal bipuramida 5. Steric number number of bond atoms number of lone pairs. Molecule has a bent t shaped structure and not a trigonal planar structure.
Trigonal system a structural category of crystalline solids sometimes considered as a subdivision of the hexagonal system. A perfect trigonal planar geometry is a kind of structure where it consists of four atoms in a molecule. The arrangement that takes place gives the molecule its geometric structure.
Trigonal planar geometries are also possible if the central atom of a compound shares double bonds with the other atoms. In chemistry trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of an equilateral triangle called peripheral atoms all in one plane. The formula used to calculate the steric number is as follows.
Trigonal planar 3 atoms around central atom tetrahedral pyramid has 4 faces 4 atoms around central atom trigonal pyramidal 3 atoms 1 pair of electrons around central atom e can go anywhere bent 2 atoms 2 pairs of electrons around central atom e can go anywhere bent 2 atoms 1 pair of electrons around. The unit cell has a single line called an axis of three fold symmetry about which the cell can be rotated by 120 degrees to produce a face identical to the face presented in the starting position.